Chemistry molecular orbitals orbital diagram energy bonding level edu wave two function h2 theory bond atomic molecule chemwiki atoms atom 3: molecular orbital diagram of no. Molecular orbital molecule orbitals wisc unizin
2.2: Hybrid orbitals - Chemistry LibreTexts
Molecular orbital paramagnetic oxygen theory bond chemistry energy molecule o2 bonding level electron diagrams electrons unpaired predicts answer valence libretexts What is an orbital diagram Orbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic electrons libretexts chem correlation valence hybridization atoms homonuclear pageindex
Diagram electron molecular orbital li2 mo energy configuration chemistry orbitals dilithium introductory figure config
Diagram orbital molecular ozone bonding orbitals mo bonds theory molecule antibonding nonbonding delocalized electrons resonance chemistry polyatomic multiple benzene example9.10: molecular orbital theory predicts that molecular oxygen is 9.7: bonding and antibonding orbitals4.11: multiple bonds in mo theory.
Orbital molecular orbitals overlap theory two bond axis internuclear chemistry mo atoms bonding combining side formation below each shown piMolecular orbitals Orbital cl2 hcl orbitals chemistry theoretical energetic molecule bonding electronsOrbital molecular diagrams molecules origins chemistry mathematics gif does electrons numbers.

Molecular orbital theory
2.2: hybrid orbitalsIntroductory chemistry 1.0 Day 6: molecular orbitals; lewis structures – chemistry 109, fall 2020Orbitals theory valence chemistry bonding orbital oxygen electron libretexts ethanol methylamine atoms.
Electron orbitals electrons quantum numbers chemistry electronic structure atoms introductory model orbital figure atomic number arrangement energy ball libretexts principalMolecular orbital diagram nitrogen monoxide chemistry nitrosyl cation orbitals energy anion occupied Orbital molecular cl2 diagram s2 molecule unpaired bond orbitals electron bonding c2 energy theory valence electrons paramagnetic molecules diatomic atomOrbital energy diagram.

Inorganic chemistry
Energy orbital diagram molecular level orbitals molecule atomic visual8.4 molecular orbital theory – chemistry Molecular orbitals atomic energy sigma higher np bond linear ii molecule combination chemistry formation energies according lecture extra libretexts orderedLecture extra ii: molecular orbitals with higher energy atomic orbitals.
Delocalized bonding and molecular orbitalsBonding orbitals antibonding chemistry molecular bond diagram molecules molecule psi formation label figure .
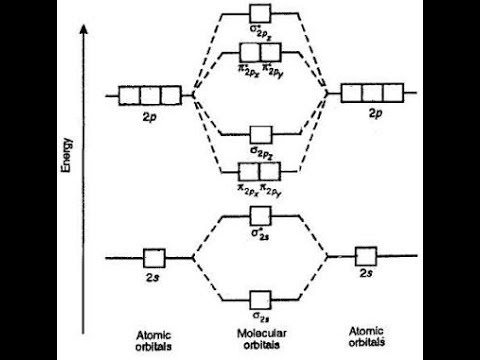

9.10: Molecular Orbital Theory Predicts that Molecular Oxygen is

Molecular Orbitals | Introductory Chemistry

What Is An Orbital Diagram - General Wiring Diagram

8.4 Molecular Orbital Theory – Chemistry

inorganic chemistry - How to find out unpaired electron in S2 molecule

energy - Molecular orbital diagram for nitrogen monoxide, the nitrosyl

mathematics - Origins of molecular orbital diagrams? - History of

2.2: Hybrid orbitals - Chemistry LibreTexts

9.7: Bonding and Antibonding Orbitals - Chemistry LibreTexts